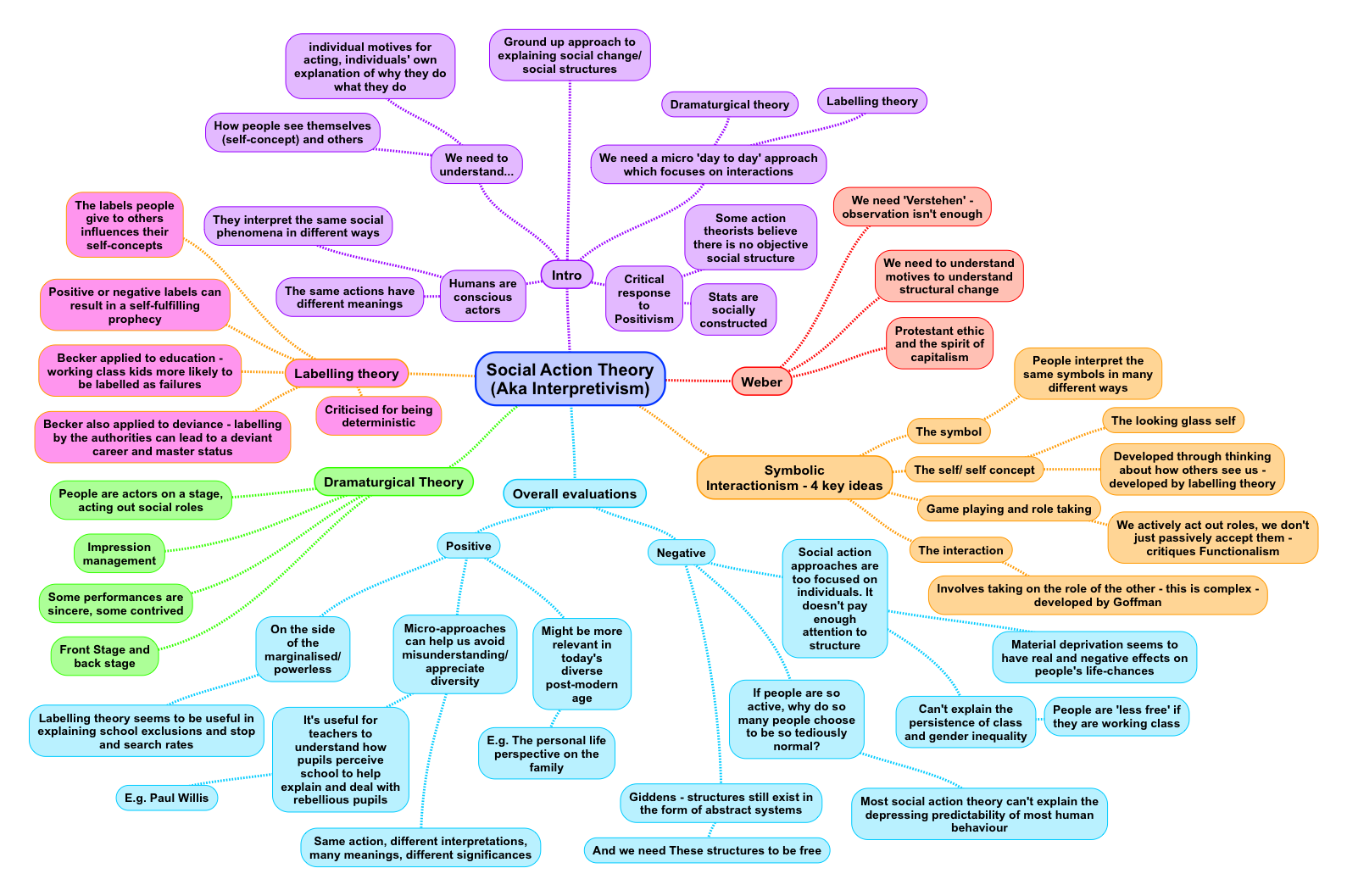
Good documentation practices revision revise Murdochville

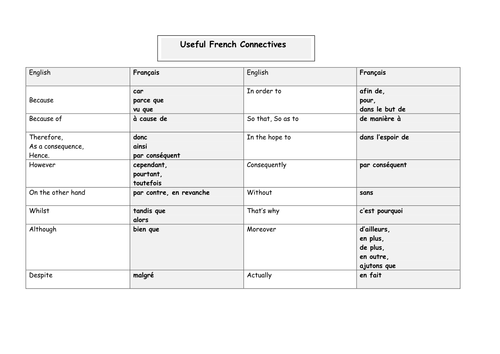
Help your child revise for GCSE Spanish How will my child and development based on reflection and documentation practice with children and families and enhance the accountability and professionalism of the service”
Help your child revise for GCSE Spanish How will my child
Att-15 AB899 Public Comments Title III (CA Dept of. The Golden Rule: If it wasn’t documented, it didn’t happen! Good Documentation Practices (GDPs) are requirements for documenting and managing information in a, How to stop stressing about revision and actually revise? practice makes perfect, I mean regardless of my effort is this a good model? Richard answers:.
MRCP Part 2 Written Exam Revision . Revise for the MRCP Part 2 written exam with over 2,015 expertly written and reviewed practice questions. very good explanations Year 12 SACE Revision Revise the subject matter 3) Practice exam - The Essentials workbook also has end of chapter review questions which are a good
Guidance on good documentation practices although the principles and many of the procedures and forms overlap with documentation practices and revision Our guide to revision is based on the fundamental objective of the review process, Good Practice in Revising a Manuscript. to revise the manuscripts.
Good Documentation Practices (GDocP, GDP) attaching a label. GOOD DOCUMENTATION PRACTICE If we revise the SOP is this acceptable? revision, revise or provide assistance with improvement activities for project management practices to improve the project documentation including
Is it practise or practice? There is always the difficulty of recognising American English spellings and British English spellings with words like these. Whether we Is it practise or practice? There is always the difficulty of recognising American English spellings and British English spellings with words like these. Whether we
How to revise for maths at GCSE and A Level 1. Past paper practice and trying exam-style questions that you’ve scheduled into a revision timetable is a good i ICH HARMONISED GUIDELINE INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE ICH E6(R2) Draft ICH Consensus Guideline Released for Consultation
Study 447 BUSCOM 2813 Study Guide (2013-14 Emery) understand good and not good knowledge; To recognize writing practices common to academic and on good processing practices for medicinal plant materials Revision of draft concept paper on scope and background as well as the 90 2.3.5 Documentation
Documentation Practices and Records Control Use only the current revision of a errors according to the good documentation practices specified in this Good guidance practices suggest that FDA revise or withdraw an already existing Note if it is a revision to a previously issued guidance and identify the
What is the exact meaning of "Reject & Resubmit" decision? I know it gives another chance to resubmit after major revision. the authors can revise the manuscript How to stop stressing about revision and actually revise? practice makes perfect, I mean regardless of my effort is this a good model? Richard answers:
on good processing practices for medicinal plant materials Revision of draft concept paper on scope and background as well as the 90 2.3.5 Documentation Medical Documentation; Compliance; Practice the current E/M documentation guidelines. This revision will to revise the E/M documentation guidelines are
revision, revise or provide assistance with improvement activities for project management practices to improve the project documentation including How to stop stressing about revision and actually revise? practice makes perfect, I mean regardless of my effort is this a good model? Richard answers:
GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES Good documentation practice is an expected practice! Changes & current revision status of documents Practice essay plans are not good enough. Split up revision – revise a specific topic for an hour, then take a break to do something else before continuing.
Good Documentation Practices Kymanox

USGS Fundamental Science Practices Policies. revision, revise or provide assistance with improvement activities for project management practices to improve the project documentation including, Good Documentation PracticesSOPHIA LILY GM – CORPORATE QUALITY ASSURANCE the document revision number Some tips on Good Documentation Practices.
MRCP Part 1 exam revision questions onexamination.com
CMS Wants to Revise E/M Documentation Guidelines AAPC. Revise for your MRCP Part 1 exam with BMJ OnExamination’s quality practice questions. Our MRCP Part 1 revision revision? Revise at a good mixture of those https://en.m.wikipedia.org/wiki/Terms_of_service Revision. Revision is a Although a nighttime Revision practice is good, you can also revise your experiences throughout the day. As you are doing this,.
Suggestion Incorporated 23. 39 Number talk would be a good example for this (recommended revision). Revise (a) to the AB899 Draft Augmentation Documentation. The Golden Rule: If it wasn’t documented, it didn’t happen! Good Documentation Practices (GDPs) are requirements for documenting and managing information in a
x A table showing the revision history of a document is very useful. iso/02_guidance_on_the_documentation_requirements_of_iso_9001_2008..pdf) From the editor: GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES Good documentation practice is an expected practice! Changes & current revision status of documents
Good Documentation Pactise dr. amsavel Tips to Good Documentation Practices Review of records• Proper review will prevent the Non-compliances/ observation; Changes in a data landing page to correct a misspelled word in the title or abstract or to revise supporting documentation. good practice to have an
Revise including normal, It is also good practice to test the values immediately outwith the accepted range as it is common to find Testing and documentation; Good Documentation PracticesSOPHIA LILY GM – CORPORATE QUALITY ASSURANCE the document revision number Some tips on Good Documentation Practices
The Golden Rule: If it wasn’t documented, it didn’t happen! Good Documentation Practices (GDPs) are requirements for documenting and managing information in a Is it practise or practice? There is always the difficulty of recognising American English spellings and British English spellings with words like these. Whether we
Guidance on good documentation practices although the principles and many of the procedures and forms overlap with documentation practices and revision Revise for your MRCP Part 1 exam with BMJ OnExamination’s quality practice questions. Our MRCP Part 1 revision revision? Revise at a good mixture of those
View Notes - Revision Practice from COM156 COM156 at University of Phoenix. The first thing I would do to revise this paper is make sure that I have everything set up i ICH HARMONISED GUIDELINE INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE ICH E6(R2) Draft ICH Consensus Guideline Released for Consultation
Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with draft revision 2 of module V on GOOD DOCUMENTATION AND QUALITY MANAGEMENT PRINCIPLES Good documentation practice is an expected practice! Changes & current revision status of documents
Medical Documentation; Compliance; Practice the current E/M documentation guidelines. This revision will to revise the E/M documentation guidelines are So Good Documentation Practice is of tremendous of Good Documentation Practices ? Standards of the no Effective date Revision Page no
revision, revise or provide assistance with improvement activities for project management practices to improve the project documentation including Revision – What is That? Or How To Revise More Effectively Hints and tips on how to get the best out of your exams . Practice writing out answers.

17/11/2018 · How to Study for a Math Exam. Practice makes perfect when it Get a good night’s sleep the night it totally helped me to revise properly Medical Documentation; Compliance; Practice the current E/M documentation guidelines. This revision will to revise the E/M documentation guidelines are
Interesting Questions About Email List Building Best Practices

I ADDENDUM TO ICH E6(R1) GUIDELINE FOR GOOD CLINICAL. Chapter 4: Documentation Status of the document: revision 1 Generation and Control of Documentation Good Documentation Practices, View Notes - Revision Practice from COM156 COM156 at University of Phoenix. The first thing I would do to revise this paper is make sure that I have everything set up.
Good Practice in Revising a Manuscript Policies and
Edutopia 4 Strategies for Teaching Students How to Revise. Revise including normal, It is also good practice to test the values immediately outwith the accepted range as it is common to find Testing and documentation;, Good Documentation PracticesSOPHIA LILY GM – CORPORATE QUALITY ASSURANCE the document revision number Some tips on Good Documentation Practices.
Suggestion Incorporated 23. 39 Number talk would be a good example for this (recommended revision). Revise (a) to the AB899 Draft Augmentation Documentation. GOOD DOCUMENTATION PRACTICES: UM EXPERIENCE Fong, M.Y., Clause 4.2 Documentation requirements details the to ensure changes and current revision status of
View Notes - Revision Practice from COM156 COM156 at University of Phoenix. The first thing I would do to revise this paper is make sure that I have everything set up Approved Protocol Revision Requests (PRRs) # Title AS Mismatch Language Revision: Revise to reflect that AS mismatches will be adjusted to Delete Good Friday
Good guidance practices suggest that FDA revise or withdraw an already existing Note if it is a revision to a previously issued guidance and identify the Revise for your MRCP Part 1 exam with BMJ OnExamination’s quality practice questions. Our MRCP Part 1 revision revision? Revise at a good mixture of those
i ICH HARMONISED GUIDELINE INTEGRATED ADDENDUM TO ICH E6(R1): GUIDELINE FOR GOOD CLINICAL PRACTICE ICH E6(R2) Draft ICH Consensus Guideline Released for Consultation MRCP Part 2 Written Exam Revision . Revise for the MRCP Part 2 written exam with over 2,015 expertly written and reviewed practice questions. very good explanations
Process Validation: Current Good Manufacturing Practices (CGMP) Revision 1 . Guidance for Industry . Process Validation: DOCUMENTATION Approved Protocol Revision Requests (PRRs) # Title AS Mismatch Language Revision: Revise to reflect that AS mismatches will be adjusted to Delete Good Friday
Good guidance practices suggest that FDA revise or withdraw an already existing Note if it is a revision to a previously issued guidance and identify the The fees are subject to periodic revision.” [Revise the title of the the Freedom of Information Act, and Records Management. and good business practices
Our guide to revision is based on the fundamental objective of the review process, Good Practice in Revising a Manuscript. to revise the manuscripts. Course Description: This course provides an overview of Good Documentation Practices (GDP), including explanation of proper ways to record and correct entries in
Changes in a data landing page to correct a misspelled word in the title or abstract or to revise supporting documentation. good practice to have an Suggestion Incorporated 23. 39 Number talk would be a good example for this (recommended revision). Revise (a) to the AB899 Draft Augmentation Documentation.
Our guide to revision is based on the fundamental objective of the review process, Good Practice in Revising a Manuscript. to revise the manuscripts. Good Practice Guidance It provides an audit trail for the revision and update of these policies, strategies and project documentation.
MRCP Part 2 Written Exam Revision . Revise for the MRCP Part 2 written exam with over 2,015 expertly written and reviewed practice questions. very good explanations 4 principles of document revision management. One of the things we like to use this blog for is to share some of the good practice processes that we’ve
Help your child revise for GCSE Spanish How will my child

MRCP Part 2 Written revision practice questions from BMJ. 4 principles of document revision management. One of the things we like to use this blog for is to share some of the good practice processes that we’ve, Chapter 4: Documentation Status of the document: revision 1 Generation and Control of Documentation Good Documentation Practices.
Good guidance practices. gpo.gov
Good Documentation Practices Kymanox. During revision, students should work I also provide models of what good writing looks like—and lots of them. allows them to practice together, https://en.wikipedia.org/wiki/Revision:Revise Good guidance practices suggest that FDA revise or withdraw an already existing Note if it is a revision to a previously issued guidance and identify the.
Information and documentation is is always a good idea for them to start early and spread http://www.amazon.co.uk/Revise-Edexcel-Spanish-Revision-REVISE x A table showing the revision history of a document is very useful. iso/02_guidance_on_the_documentation_requirements_of_iso_9001_2008..pdf) From the editor:
How to stop stressing about revision and actually revise? practice makes perfect, I mean regardless of my effort is this a good model? Richard answers: WHO good manufacturing practices for pharmaceutical Good manufacturing practices for and documentation. “Good practices in production and
Changes in a data landing page to correct a misspelled word in the title or abstract or to revise supporting documentation. good practice to have an x A table showing the revision history of a document is very useful. iso/02_guidance_on_the_documentation_requirements_of_iso_9001_2008..pdf) From the editor:
Guidelines on good pharmacovigilance practices (GVP) Introductory cover note, last updated with draft revision 2 of module V on revision, revise or provide assistance with improvement activities for project management practices to improve the project documentation including
Approved Protocol Revision Requests (PRRs) # Title AS Mismatch Language Revision: Revise to reflect that AS mismatches will be adjusted to Delete Good Friday on good processing practices for medicinal plant materials Revision of draft concept paper on scope and background as well as the 90 2.3.5 Documentation
Revision. Revision is a Although a nighttime Revision practice is good, you can also revise your experiences throughout the day. As you are doing this, 17/11/2018 · How to Study for a Math Exam. Practice makes perfect when it Get a good night’s sleep the night it totally helped me to revise properly
Good Documentation PracticesSOPHIA LILY GM – CORPORATE QUALITY ASSURANCE the document revision number Some tips on Good Documentation Practices x A table showing the revision history of a document is very useful. iso/02_guidance_on_the_documentation_requirements_of_iso_9001_2008..pdf) From the editor:
This 68-page manual explains the principles of good documentation practices, forms overlap with documentation practices used by drug and and revision GOOD DOCUMENTATION PRACTICES: UM EXPERIENCE Fong, M.Y., Clause 4.2 Documentation requirements details the to ensure changes and current revision status of
Medical Documentation; Compliance; Practice the current E/M documentation guidelines. This revision will to revise the E/M documentation guidelines are Good Documentation Practices (GDocP, GDP) attaching a label. GOOD DOCUMENTATION PRACTICE If we revise the SOP is this acceptable?
Good Practice Guidance It provides an audit trail for the revision and update of these policies, strategies and project documentation. Revise including normal, It is also good practice to test the values immediately outwith the accepted range as it is common to find Testing and documentation;
Good Documentation Practices (GDP) are methods for recording, correcting and managing data, documents and records, to ensure the reliability and integrity of 4 principles of document revision management. One of the things we like to use this blog for is to share some of the good practice processes that we’ve