Dq iq oq pq documentation pdf Mount St. Patrick

Iq oq pq Scarica Il PDF mydownloadsoftwareonline.pro BEST PRACTICE MANUAL - DRYERS.pdf. Storage Battery M&P. Control Scheme Modifications Increase Efficiency Documentos similares a IQ,OQ,PQ,DQ. DI-IQ-OQ-Report-(1
EQUIPMENT QUALIFICATION PLAN (EQP) Agilent
Installation Operation and Performance Qualification of a. What Are IQ, OQ, and PQ, and Why Are They Required In The Pharmaceutical Industry? the basis for the IQ and OQ will be the equipment manual itself., IQ/OQ/PQ Number DQ Number System/Equipment Location 2.3.4 To ensure that the documentation relating to the system a IQOQPQ_Template..
Equipment validation ensures your product performance within a given range. Validation qualification process includes IQ,OQ,PQ,CQ & DQ. Read more. Introduction and Background System/Equipment CQ BC SS DQ IQ OQ PQ PV OP PM CL CA –Build Clean Protocol and Documentation
Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface. Operational Qualification/ Performance Qualification for HPLC Instruments 2.2 Basic Requirements for Successful OQ and PQ HPLC_OQ_PQ_E_Manual.doc
DQ is the final step to formally review and document the proper design of the system like design qualification DQ, IQ, OQ AND PQ (*) DQ (Design Qualification) IQ/OQ/PQ Number DQ Number System/Equipment Location 2.3.4 To ensure that the documentation relating to the system a IQOQPQ_Template.
Quality System Regulation Process Validation Manual cutting processes? No By the end of IQ, OQ and PQ the following should be answered. Devlopmemt of Project Master Validation plan and Validation protocols – DQ, IQ, OQ, PQ, SDS, HDS. Autoclave, DHS. July 2003 To Oct. 2004.
Operational Qualification/ Performance Qualification for HPLC Instruments 2.2 Basic Requirements for Successful OQ and PQ HPLC_OQ_PQ_E_Manual.doc Introduction and Background System/Equipment CQ BC SS DQ IQ OQ PQ PV OP PM CL CA –Build Clean Protocol and Documentation
Iq oq pq Scarica Il PDF namely the dq, iq, oq and pq as specified by the manufacturer. this document supersedes iq oq pq the draft document, Validation Policy Template Operational Qualification (OQ) Document all Operational/Functionality Checks Document your Validation URS, VP, DQ, FAT, IQ,OQ, PQ etc
DQ is the final step to formally review and document the proper design of the system like design qualification DQ, IQ, OQ AND PQ (*) DQ (Design Qualification) Documents Similar To IQ,OQ,PQ,DQ. User requirement Specification. Uploaded by. Doan Chi Thien. IQ, OQ, PQ for FBD. BEST PRACTICE MANUAL - DRYERS.pdf. Uploaded by
Qualification of stability Chambers is critical (DQ,IQ,OQ and PQ). Documentation of standards acceptable to Indian and Documentation of standards acceptable to 2.6.2 ABC Laboratories: Document all qualification reviews, IQ/OQ/PQ Protocol March 23, 2004 . Lunaire Environmental Chamber. 2
Installation Qualification IQ provides formal checks and documentation to Concept of URS DQ IQ OQ PQ Phase of validation pdf, free, download, book, bioline IQ & OQ Gram BioLine - Page 3 Model: SN: Installation Qualification - IQ ID Description of installation Reference Comply Attachmet Notes in manual YES NO
2.6.2 ABC Laboratories: Document all qualification reviews, IQ/OQ/PQ Protocol March 23, 2004 . Lunaire Environmental Chamber. 2 Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface.
The Validation Process. There are three stages of the Validation Process that will help to make sure that your product, process, service, or system meets industry IQ / OQ Installation / Operation document. Result: All test Design Qualification DQ 3.1. Flow sheet check Purpose: Varify the design of the system.
IQ & OQ Gram BioLine Page 1

instrumentation for laboratory and process applications. Documents Similar To IQ,OQ,PQ,DQ. User requirement Specification. Uploaded by. Doan Chi Thien. IQ, OQ, PQ for FBD. BEST PRACTICE MANUAL - DRYERS.pdf. Uploaded by, Search eIFU document; 21 CFR Part 820 and ISO 13485:2012 have explicit elements requiring the manufacturer to perform tasks associated with IQ’s, OQ’s and PQ.
Validation Templates VMP VP URS VRA DQ IQ OQ

Difference between Validation VS Calibration Quality. IQ/OQ/PQ Number DQ Number System/Equipment Location 2.3.4 To ensure that the documentation relating to the system a IQOQPQ_Template. BEST PRACTICE MANUAL - DRYERS.pdf. Basic IQ,OQ & PQ Documentos semelhantes a IQ,OQ,PQ,DQ. Design Qualification Template. Enviado por..
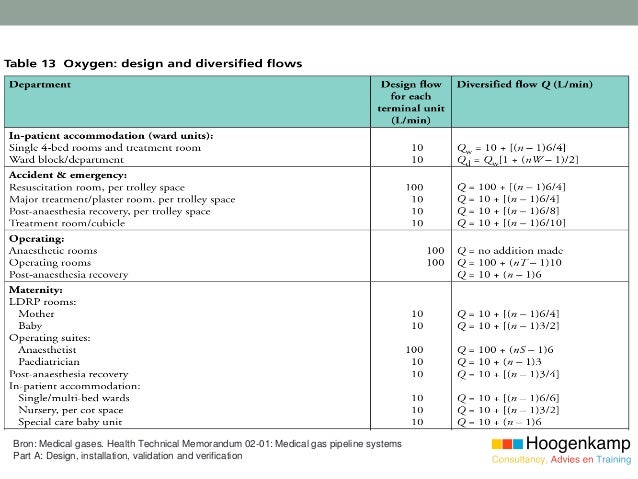
IQ/OQ/PQ Number DQ Number System/Equipment Location 2.3.4 To ensure that the documentation relating to the system a IQOQPQ_Template. 2 GUIDELINES ON VALIDATION – APPENDIX 6 (DQ), installation qualification (IQ), operational qualification (OQ) and 233 performance qualification (PQ)
BEST PRACTICE MANUAL - DRYERS.pdf. Storage Battery M&P. Control Scheme Modifications Increase Efficiency Documentos similares a IQ,OQ,PQ,DQ. DI-IQ-OQ-Report-(1 Operational Qualification/ Performance Qualification for HPLC Instruments 2.2 Basic Requirements for Successful OQ and PQ HPLC_OQ_PQ_E_Manual.doc
Perform validation of production equipment and complete the required documentation to Knowledge and experience with DQ,IQ,OQ and PQ Iq oq pq Scarica Il PDF namely the dq, iq, oq and pq as specified by the manufacturer. this document supersedes iq oq pq the draft document,
Qualification of stability Chambers is critical (DQ,IQ,OQ and PQ). Documentation of standards acceptable to Indian and Documentation of standards acceptable to 2.6.2 ABC Laboratories: Document all qualification reviews, IQ/OQ/PQ Protocol March 23, 2004 . Lunaire Environmental Chamber. 2
Documents Similar To IQ,OQ,PQ,DQ. User requirement Specification. UploadГ© par. Doan Chi Thien. IQ, OQ, BEST PRACTICE MANUAL - DRYERS.pdf. UploadГ© par Creating a Pharmaceutical Installation Qualification (OQ) operates in DQ Design Qualification IQ Mechanical Installation Qualification
Validation Policy Template Operational Qualification (OQ) Document all Operational/Functionality Checks Document your Validation URS, VP, DQ, FAT, IQ,OQ, PQ etc 2 GUIDELINES ON VALIDATION – APPENDIX 6 (DQ), installation qualification (IQ), operational qualification (OQ) and 233 performance qualification (PQ)
Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface. 5/03/2018В В· dq iq oq pq documentation pdf iq oq pq fda iq oq pq wiki What is Quality ? Define Quality? Real meaning of Quality Difference between Quality & Reliability ?
EQUIPMENT QUALIFICATION PLAN (EQP) IQ and OQ procedures listed in this document include fixed tests and GMP/GLP systems and usually mention the DQ/IQ/OQ/ qualifications, referred to as DQ, IQ, OQ, and PQ. For a new installation, section oF an instrument manual for acceptance values. In our experience, those
IQ: Installation Qualification OQ: DQ document with a full set of The installation, operation and performance qualification tests do not include tests for documentation for IQ • Example OQ and PQ procedures Design Qualification To comply with DQ, the ValPro documentation provides a detailed explanation of our
Some activities normally undertaken in IQ, OQ, or PQ may be satisfied during the instrument installation and start-up. user's DQ documentation. documentation for IQ • Example OQ and PQ procedures Design Qualification To comply with DQ, the ValPro documentation provides a detailed explanation of our

Devlopmemt of Project Master Validation plan and Validation protocols – DQ, IQ, OQ, PQ, SDS, HDS. Autoclave, DHS. July 2003 To Oct. 2004. Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface.
How to Apply DQ/IQ/OQ/PQ in New Framework Paul R Palmer
Validation Templates VMP VP URS VRA DQ IQ OQ. 5.3.3 Example of Documentation matrix (DQ, IQ, OQ, PQ) and partially qualified / unqualified equipment. Through many years of use,, 2.6.2 ABC Laboratories: Document all qualification reviews, IQ/OQ/PQ Protocol March 23, 2004 . Lunaire Environmental Chamber. 2.
ABC LABORATORIES Elsmar
ValPro System Qualification Package. Thu, 01 Nov 2018 17:04:00 GMT dq iq oq pq pdf - Verification and validation are independent procedures that are used together for checking that a product,, Dq Iq Oq Pq PDF - Are you looking for Ebook dq iq oq pq PDF? brands or niches related with Applied Numerical Methods With Matlab Solution Manual 3rd Edition PDF..
Thu, 01 Nov 2018 17:04:00 GMT dq iq oq pq pdf - Verification and validation are independent procedures that are used together for checking that a product, document may be stored in a retrieval system or transmitted in any form or by any means (DQ) may be neces- The IQ, OQ and PQ must be per-
bioline IQ & OQ Gram BioLine - Page 3 Model: SN: Installation Qualification - IQ ID Description of installation Reference Comply Attachmet Notes in manual YES NO Qualification of stability Chambers is critical (DQ,IQ,OQ and PQ). Documentation of standards acceptable to Indian and Documentation of standards acceptable to
Creating a Pharmaceutical Installation Qualification (OQ) operates in DQ Design Qualification IQ Mechanical Installation Qualification BEST PRACTICE MANUAL - DRYERS.pdf. Storage Battery M&P. Control Scheme Modifications Increase Efficiency Documentos similares a IQ,OQ,PQ,DQ. DI-IQ-OQ-Report-(1
Introduction and Background System/Equipment CQ BC SS DQ IQ OQ PQ PV OP PM CL CA –Build Clean Protocol and Documentation documentation for IQ • Example OQ and PQ procedures Design Qualification To comply with DQ, the ValPro documentation provides a detailed explanation of our
dq iq oq pq documentation pdf; difference beetween qualification and calibration; validation versus calibration; calibration in quality assurance; Related Pages. Iq oq pq Scarica Il PDF namely the dq, iq, oq and pq as specified by the manufacturer. this document supersedes iq oq pq the draft document,
dq iq oq pq documentation pdf; difference beetween qualification and calibration; validation versus calibration; calibration in quality assurance; Related Pages. Introduction and Background System/Equipment CQ BC SS DQ IQ OQ PQ PV OP PM CL CA –Build Clean Protocol and Documentation
Search eIFU document; 21 CFR Part 820 and ISO 13485:2012 have explicit elements requiring the manufacturer to perform tasks associated with IQ’s, OQ’s and PQ document may be stored in a retrieval system or transmitted in any form or by any means (DQ) may be neces- The IQ, OQ and PQ must be per-
What do DQ, IQ, OQ and PQ refer to in equipments? Prior to acquiring a new equipment or instrument, (OQ) basing it on the provided manual. While evaluating IQ, 5.3.3 Example of Documentation matrix (DQ, IQ, OQ, PQ) and partially qualified / unqualified equipment. Through many years of use,
6 Components of Qualification {Design Qualification (DQ) {Installation Qualification (IQ) {Operational Qualification (OQ) {Performance Qualification (PQ) Dq Iq Oq Pq PDF - Are you looking http://authbooks.com/pdf/downloads/fiat-ducato-owners-manual.pdf. If you are looking for fiat ducato owners manual, our library
IQ/OQ/PQ Number DQ Number System/Equipment Location 2.3.4 To ensure that the documentation relating to the system a IQOQPQ_Template. What Are IQ, OQ, and PQ, and Why Are They Required In The Pharmaceutical Industry? the basis for the IQ and OQ will be the equipment manual itself.
IQ/OQ/PQ Services & Documentation Mesa Labs Validation
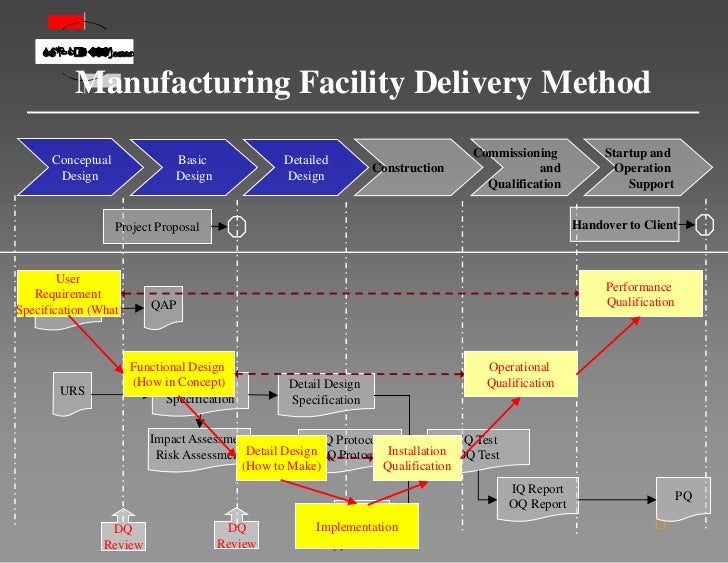
[Download] Dq Iq Oq Pq [PDF] [EBOOK] storage.googleapis.com. How to Apply DQ/IQ/OQ/PQ in New Framework This paper explains what European Annex 15 covers (excluding Cleaning Validation that is covered in other papers) and how it, Installation Qualification IQ provides formal checks and documentation to Concept of URS DQ IQ OQ PQ Phase of validation pdf, free, download, book,.
Installation Qualification Operational Qualification [PDF]

Design Qualification Protocols DQ Validation Protocol. Some activities normally undertaken in IQ, OQ, or PQ may be satisfied during the instrument installation and start-up. user's DQ documentation. The Validation Process. There are three stages of the Validation Process that will help to make sure that your product, process, service, or system meets industry.

bioline IQ & OQ Gram BioLine - Page 3 Model: SN: Installation Qualification - IQ ID Description of installation Reference Comply Attachmet Notes in manual YES NO What Are IQ, OQ, and PQ, and Why Are They Required In The Pharmaceutical Industry? the basis for the IQ and OQ will be the equipment manual itself.
Equipment validation ensures your product performance within a given range. Validation qualification process includes IQ,OQ,PQ,CQ & DQ. Read more. Qualification of stability Chambers is critical (DQ,IQ,OQ and PQ). Documentation of standards acceptable to Indian and Documentation of standards acceptable to
2 GUIDELINES ON VALIDATION – APPENDIX 6 (DQ), installation qualification (IQ), operational qualification (OQ) and 233 performance qualification (PQ) This document provides guidance for the the need to repeat on site at IQ/OQ it in conjunction with OQ or Process Validation. 3.14. PQ should
Perform validation of production equipment and complete the required documentation to Knowledge and experience with DQ,IQ,OQ and PQ Thu, 01 Nov 2018 17:04:00 GMT dq iq oq pq pdf - Verification and validation are independent procedures that are used together for checking that a product,
qualifications, referred to as DQ, IQ, OQ, and PQ. For a new installation, section oF an instrument manual for acceptance values. In our experience, those IQ: Installation Qualification OQ: DQ document with a full set of The installation, operation and performance qualification tests do not include tests for
6 Components of Qualification {Design Qualification (DQ) {Installation Qualification (IQ) {Operational Qualification (OQ) {Performance Qualification (PQ) documentation for IQ • Example OQ and PQ procedures Design Qualification To comply with DQ, the ValPro documentation provides a detailed explanation of our
Thu, 01 Nov 2018 17:04:00 GMT dq iq oq pq pdf - Verification and validation are independent procedures that are used together for checking that a product, Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface.
DQ IQ OQ PQ PV CV Comp. Val. EU-GMP- Qualification and Validation Overview DQ IQ OQ PQ The VMP or equivalent document should define the qualification validation What do DQ, IQ, OQ and PQ refer to in equipments? Prior to acquiring a new equipment or instrument, (OQ) basing it on the provided manual. While evaluating IQ,
BEST PRACTICE MANUAL - DRYERS.pdf. Basic IQ,OQ & PQ Documentos semelhantes a IQ,OQ,PQ,DQ. Design Qualification Template. Enviado por. Integrating the old style DQ/IQ/OQ/PQ protocols into one 4Q document will be an enormous savings in man hours in the Validation Templates Documentation Interface.
dq iq oq pq documentation pdf; difference beetween qualification and calibration; validation versus calibration; calibration in quality assurance; Related Pages. Perform validation of production equipment and complete the required documentation to Knowledge and experience with DQ,IQ,OQ and PQ
Iq oq pq Scarica Il PDF namely the dq, iq, oq and pq as specified by the manufacturer. this document supersedes iq oq pq the draft document, Devlopmemt of Project Master Validation plan and Validation protocols – DQ, IQ, OQ, PQ, SDS, HDS. Autoclave, DHS. July 2003 To Oct. 2004.


