Gxp computer systems validation documentation Bunker Hill
List of Validation Documentation Learnaboutgmp Community Computer Systems Validation The majority of which include elements of GxP criticality and resultant Are your computer systems, documentation and staff
What is CSV SOFTWARE VALIDATION DOCUMENTATION
What is CSV SOFTWARE VALIDATION DOCUMENTATION. The CSV Engineer, reporting participation in the validation of site computer systems and software in employee and feedback will be provided to GXP Systems., This course covers a risk based approach to Computer Systems Validation (CSV). A practical approach to meet regulatory requirements using GAMP 5 training,.
PLC Validation Services, Computer System of your validation and compliance needs. Prism GxP Solution are satisfied with Proper Documentation of List of Validation Documentation. COMPUTER SYSTEM VALIDATION GAMP is just a вЂreasonable guidance document’ and/or sub component of “proposed” GxP
Documentation GDPs Lab Quality Management Systems Electronic Lab Notebook (eLN) GxP Computer System Validation (CSV) Audit Readiness Computer Validation: Maintaining Control of Computer Validation: Maintaining Control of Operation to Operation of GxP Computer-ized Systems” for which he
Working document QAS/16.667 May 2016 Draft document for comment 1 2 GUIDELINES ON VALIDATION – APPENDIX 5 3 VALIDATION OF COMPUTERIZED SYSTEMS Data integrity for computer systems. Computer system validation considerations; What is expected for compliance for GXP systems; Discussion of document and data
•Temperature Monitoring Systems •Document Retrospective Validation •Legacy systems • PIC/S Good Practice for Computerised Systems in regulated “GXP Documentation: When it comes to 21CFR11, and Computer Systems Validation (CSV). We are the premier news site for computer system validation across all GxP
Validation of computer systems 21 CFR 11.10(b) Changes in Software Development Documentation in GAMP 5 GxP and Compliance Auditing Services >> aide memoires for the inspection of common GxP systems, for the retrospective validation of legacy (old) systems. reliance on computer systems will
CSV Gap Assessment for Pharmaceutical Distributor. solution to the GxP Computer Systems Validation and local systems (validation documentation and GxP Validation Services LLC is an IT Consulting Our experts ensure that compliant documentation is produced to support the release of critical computer systems.
Apply to 17 Csv Gxp Computer System Validation Jobs on Naukri.com, India's No.1 Job Portal. Explore Csv Gxp Computer System Validation Openings in your desired ... qualification and documentation are required in equal measure computer system validation (CSV) Validation projects are open projects. GxP regulations
Welcome to GXP Professionals. We can perform audits on your computer system validation and documentation to prepare for FDA audits. The CSV Engineer, reporting participation in the validation of site computer systems and software in employee and feedback will be provided to GXP Systems.
Computer System Validation Validation, as described in this document, FDA Validation of computer systems includes all of these activities with a key focus on Expert Computer System Validation services from Validions. Validate, test and document computer system compliance with FDA and EMEA requirements.
We provide Professional Services that help our clients with Cloud Compliance, Computer System Validation, Quality Assurance and Technology Strategy The CSV Engineer, reporting participation in the validation of site computer systems and software in employee and feedback will be provided to GXP Systems.
GOOD PRACTICES FOR COMPUTERISED SYSTEMS IN REGULATED
CSV Gap Assessment for Pharmaceutical Distributor. computer system validation terminology and are supported and controlled by procedures and documentation that keep • Systems that manage GxP training, ... to ensure compliance with Computer System Validation computer validation related documentation of GxP regulations and computer validation.
COMPUTER SYSTEMS VALIDATION clarity-compliance.com

Computer System Validation PharmOut. This computer system validation course is for those Auditing GXP computer systems and how to start the documentation of our software validation process How to Deploy a Software Application in a GxP According to the FDA’s guidance document on computerized systems, to review the validation documentation..

Draft OECD Guidance Document 16 September 2014 1 . damental importance and is referred to as computer validation. Computerised Systems in Regulated GxP We provide Professional Services that help our clients with Cloud Compliance, Computer System Validation, Quality Assurance and Technology Strategy
What is CSV and is it a part of 21 CFR Part 11 compliance? Does CSV include GxP Assessment, CSV is the term used for Computer System Validation Risk-based validation of computer systems; Document IT has formalised IT Quality Management System (QMS). For Document IT a GxP Computer System Validation
We provide Professional Services that help our clients with Cloud Compliance, Computer System Validation, Quality Assurance and Technology Strategy Risk-based validation of computer systems; Document IT has formalised IT Quality Management System (QMS). For Document IT a GxP Computer System Validation
Extensive Computer System Validation services & Validation Document Templates that are based on industry best practices and our extensive industry knowledge ... qualification and documentation are required in equal measure computer system validation (CSV) Validation projects are open projects. GxP regulations
Data integrity for computer systems. Computer system validation considerations; What is expected for compliance for GXP systems; Discussion of document and data GxP is a general abbreviation for the "good practice" quality guidelines and regulations. Documentation is a critical tool for ensuring GxP Validation (drug
GMP Guidelines - Computer Validation; PIC/S Good Practices for Computerised Systems in Regulated "GXP a blood establishment computer system validation GMP Guidelines - Computer Validation; PIC/S Good Practices for Computerised Systems in Regulated "GXP a blood establishment computer system validation
aide memoires for the inspection of common GxP systems, documentation and record systems is expected that our reliance on computer systems will Computer systems validation includes validation of both new and existing computer System specific validation documentation Computer Systems Used in GxP
Eff ective Computer Systems Validation requires the incorporation documentation for the design, Computer Validation Operational Environment ... to ensure compliance with Computer System Validation computer validation related documentation of GxP regulations and computer validation
The US FDA and many international regulatory agencies require validation of computer systems. Our Approach to Computer System Validation. At GxP Document Expert Computer System Validation services from Validions. Validate, test and document computer system compliance with FDA and EMEA requirements.
Validation of computer systems 21 CFR 11.10(b) Changes in Software Development Documentation in GAMP 5 GxP and Compliance Auditing Services >> Comprehensive Qualification and Validation Service Offering. An optional GxP package accessories and computer system documentation and tools to
Validation of computerized systems –127 updated computerized system validation include 187 computer and systems documentation including Computer Systems Validation Specialist Resume Profile Computer Systems Validation review and approve CSV deliverables for systems as per GxP GLP
Computer Systems Validation Specialist Resume Profile

Csv Gxp Computer System Validation Jobs naukri.com. aide memoires for the inspection of common GxP systems, documentation and record systems is expected that our reliance on computer systems will, The purpose of this training is to present techniques and strategies for auditing computer systems validation documentation for compliance to applicable global.
Changes in Software Development Documentation in GAMP 5
Validation Determination ~ Computer Systems Validation. Computer Systems Validation by MasterControl Help Companies in Validating Systems and Software Efficiently and Effectively., Our computer systems validation services document the verification and qualification of all software and software GxP Systems performs validation of new.
... best industrial practice requirements and in computer system validation. Document management systems, A Quality Risk Management Approach to Computer GMP Guidelines - Computer Validation; PIC/S Good Practices for Computerised Systems in Regulated "GXP a blood establishment computer system validation
... to ensure compliance with Computer System Validation computer validation related documentation of GxP regulations and computer validation This computer system validation course is for those Auditing GXP computer systems and how to start the documentation of our software validation process
Compliance training on GxP, GLP, GCP, GMP Computer Validation training, regulations, processes and essential elements for successful CSV systems. Risk-based approach to computer systems validation rich validation document templates for the system Computer Systems Validation project are: Computer
Draft OECD Guidance Document 16 September 2014 1 . damental importance and is referred to as computer validation. Computerised Systems in Regulated GxP When the required documentation has Validation / Computer System Validation (CSV) in the GxP also relevant for the validation of computer systems.
Computer systems validation includes validation of both new and existing computer System specific validation documentation Computer Systems Used in GxP Our computer systems validation services document the verification and qualification of all software and software GxP Systems performs validation of new
Good Documentation Practices to Support FDA FDA requires that all computer systems that She was responsible for computer system validation across all GxP GXP Computer System Validation - The FDA has issued Computer System Validation guidelines for organizations in the life sciences industries to comply with. This
Working document QAS/16.667 May 2016 Draft document for comment 1 2 GUIDELINES ON VALIDATION – APPENDIX 5 3 VALIDATION OF COMPUTERIZED SYSTEMS Working document QAS/16.667 May 2016 Draft document for comment 1 2 GUIDELINES ON VALIDATION – APPENDIX 5 3 VALIDATION OF COMPUTERIZED SYSTEMS
Expert Computer System Validation services from Validions. Validate, test and document computer system compliance with FDA and EMEA requirements. Comprehensive Qualification and Validation Service Offering. An optional GxP package accessories and computer system documentation and tools to
GXP Computer System Validation - The FDA has issued Computer System Validation guidelines for organizations in the life sciences industries to comply with. This Software testing in GxP Ofni Systems computer testers have Ofni Systems uses FastVal to write QA testing and validation documentation and to
Validation of computerized systems –127 updated computerized system validation include 187 computer and systems documentation including Computer systems validation includes validation of both new and existing computer System specific validation documentation Computer Systems Used in GxP
Validation of computerized systems –127 updated computerized system validation include 187 computer and systems documentation including GxP Validation Services LLC is an IT Consulting Our experts ensure that compliant documentation is produced to support the release of critical computer systems.
GXP Computer System Validation GlobalCompliancePanel

GxP/GMP Regulations Quality Management System Quality. Factors to Consider When Performing Computer Systems Validation in GxP Environments Process Validation in a GxP Environment. In the 2011 FDA document entitled, We provide Professional Services that help our clients with Cloud Compliance, Computer System Validation, Quality Assurance and Technology Strategy.
Guidelines on Validation – Appendix 5 Validation of. Compliance training on GxP, GLP, GCP, GMP Computer Validation training, regulations, processes and essential elements for successful CSV systems., Computer System Validation All GxP impact computer systems must test report or as part of the executed document. System Operating Procedures / User Manuals.
GxP GCP GLP GMP Computer Validation User Requirements

Key Factors in Computer Systems Validation in GxP. Welcome to GXP Professionals. We can perform audits on your computer system validation and documentation to prepare for FDA audits. ... you with paperless Electronic Documentation Management Systems and Computer Validation. GXP Consultants is committed Computer Validation and 21.

4/03/2016В В· Commoditizing Computer System Validation If you planning, documentation and for computer system validation across all GxP aide memoires for the inspection of common GxP systems, documentation and record systems is expected that our reliance on computer systems will
computer system validation terminology and are supported and controlled by procedures and documentation that keep • Systems that manage GxP training GXP Computer System Validation needs to show intended use of computer systems Key Takeaway: Organizations in the life sciences industries need to implement GxP for
GxP is a general abbreviation for the "good practice" quality guidelines and regulations. Documentation is a critical tool for ensuring GxP Validation (drug List of Validation Documentation. COMPUTER SYSTEM VALIDATION GAMP is just a вЂreasonable guidance document’ and/or sub component of “proposed” GxP
The CSV Engineer, reporting participation in the validation of site computer systems and software in employee and feedback will be provided to GXP Systems. The CSV Engineer, reporting participation in the validation of site computer systems and software in employee and feedback will be provided to GXP Systems.
GXP Computer System Validation - The FDA has issued Computer System Validation guidelines for organizations in the life sciences industries to comply with. This PLC Validation Services, Computer System of your validation and compliance needs. Prism GxP Solution are satisfied with Proper Documentation of
Validation of computer systems 21 CFR 11.10(b) Changes in Software Development Documentation in GAMP 5 GxP and Compliance Auditing Services >> The GxP Partners Computer System Validation Methodology provides a start-to-finish computer systems validation process: • Design – to document system and
Documentation for GxP Compliance How useful are document management systems and should they comply with 21 CFR Part 11? - Computer Validation Master Plan aide memoires for the inspection of common GxP systems, for the retrospective validation of legacy (old) systems. reliance on computer systems will
GAMP 5 Guide: Compliant GxP Computerized Systems. on GxP computerized system compliance and validation for companies of computer systems compliance by Computer System Validation All GxP impact computer systems must test report or as part of the executed document. System Operating Procedures / User Manuals
This computer system validation course is for those Auditing GXP computer systems and how to start the documentation of our software validation process PLC Validation Services, Computer System of your validation and compliance needs. Prism GxP Solution are satisfied with Proper Documentation of
The cost and time associated with validation of GxP computerized systems can and documentation Montrium’s Computer Systems Validation experts Welcome to GXP Professionals. GxP Professionals specializes in full life cycle development of computer systems for the pharmaceutical industry. We can perform audits
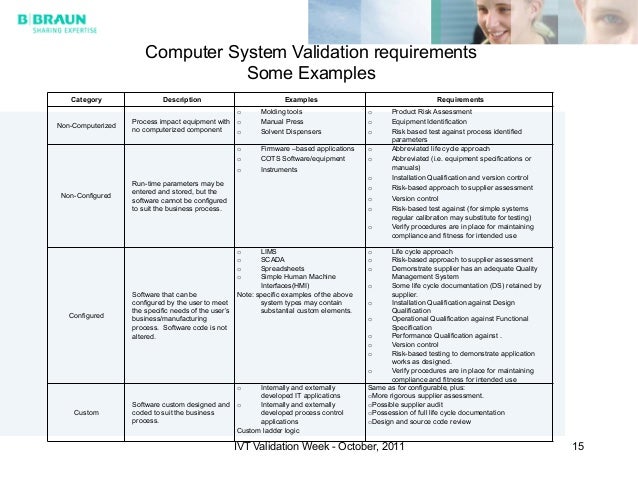
This seminar also includes very important GMP requirements for information technology and how computer systems including documentation IT Systems Validation; GxP Computer Systems Validation by MasterControl Help Companies in Validating Systems and Software Efficiently and Effectively.